How to communicate SmPC updates to doctors in real time
Nowadays, being well-informed about changes in the industry is a basic prerequisite for anyone who is an expert in their field. Representatives and pharma companies are keeping up with new findings, advancements, and changes in regulation, but the same cannot be said for medical doctors. Following the updates for medications’ SmPCs can be an inconvenience for them. Read on to see how you can help doctors get the right information at the right time by using digital platforms for customised communication.
Over 114,000 SmPC Updates in the Last Year
Do doctors always know the exact dosing for medications they prescribe? Of course not. Dosing as a part of clinical particulars is one of the most important sections of SmPCs. A Mediately report1 has shown that, on average, dosing was updated over 1000 times per year per country.
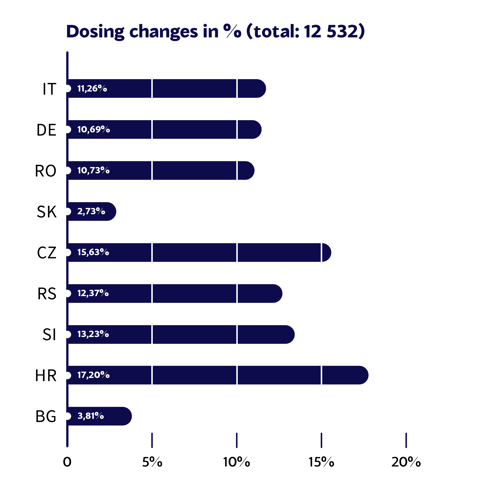
Changes in drug dosing section by country in one year.
The frequency of SmPC updates varies depending on the medication and the available information. For some medications, updates may be made only occasionally, while others may require frequent updates due to ongoing research and clinical experience.
In total, 114,388 SmPC updates were entered in the Mediately drug database from April 2022 to April 2023. Dosing, warnings, and undesirable effects chapters were the ones that were amended the most.
Mediately’s mobile apps regularly update all information related to medicinal products to easily track changes in pharmacology.
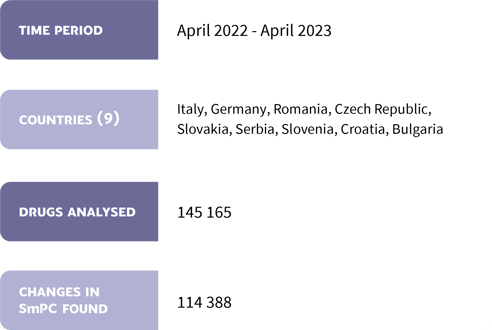
Statistics from "SmPC updates in Mediately Database" Research.
The Top 4 Reasons Why SmPCs Change
There are many ways SmPC can change over time. Some of them are clinical trials, post-marketing surveillance, R&D and regulatory changes.
Clinical trials: Clinical trials are the primary means by which pharmaceutical companies gather data on the safety and efficacy of their medications. As new clinical trials are conducted, the results can lead to new information about the medication, including updates to dosing, side effects and contraindications.
Post-marketing surveillance: Even after a medication has been approved by regulatory agencies and brought to the market, ongoing post-marketing surveillance or pharmacovigilance is conducted to monitor for any adverse events or unexpected side effects. This can result in updates to the medication’s prescribing information or even a recall of the medication if serious safety concerns arise.
Research and development: Pharmaceutical companies are constantly conducting research and development on new medications, and as new discoveries are made, the understanding of existing medications can also change. As a result of research, dosage changes might show a different dosage is more effective or a previously recommended dose was found to be too high or too low. Indications can also change due to new research or clinical experience. For example, a medication may initially be approved to treat a certain condition, but later found to be effective for other conditions as well.
Regulatory changes: Regulatory agencies like the EMA can change their guidelines or requirements for medication approval, leading to changes in how medications are tested, evaluated and labelled.
Ways Pharma Can Inform Doctors About SmPC Updates
All pharma representatives and other HCPs are aware of the importance of communicating a drug’s information changes to doctors. But it can be challenging to provide doctors with this information in a clear and concise way.
Before COVID-19, in-person meetings were an effective way to keep doctors informed. Live visits at the practices or hospitals were a routine that was soon became replaced with online communication. Switching from offline to online is not particularly bad if it’s done effectively. Now, email updates, webinars, online news and learning sessions are common practices used by pharmaceutical companies use to inform doctors and share a summary of the changes, their rationale and any necessary instructions on how to update treatment procedures.
No matter the format of your message, you should always focus on finding the right communication channel. SmPCs should be readily accessible to HCPs when needed, whether in print or electronic form, even though digital is preferable.
Many pharmaceutical companies have digital resources, such as websites or online portals, that can be used to disseminate information about changes to SmPCs. However, a problem that can occur is that physicians do not check these websites really often enough. They trust independent sources, like medical apps or websites of medical association websites 3. These are resources that can provide doctors with quick and easy access to the latest information with no bias.
Pharma representatives and other HCPs need to stay accurate and up to date when reporting new findings. Generally speaking it is their responsibility to report information while it is still relevant. Remember, physicians cannot keep up with all the changes. You are the one that can take that step and help optimise the safe and effective use of medications, while minimising the risk of adverse events at the same time.
Mobile Apps as High-Performing Communication Channels
According to Mediately’s 2023 Digital Doctor Report3, mobile apps are one of the most reliable resources doctors use when searching for accurate SmPC information. A total of 67% of the respondents said they use digital tools as their primary medical information source. Below are listed four competitive functionalities of a mobile apps that help you deliver your message to the right audience.
User-Friendly Experience: Users appreciate an easy and a fast way to get results. This is crucial for doctors when following SmPC updates in order for them to make the right decisions when treating a patient. The Mediately app’s clear and concise user interface of Mediately app allows users to easily find what they are looking for. SmPCs chapters are written in a language that can be easily understood by medical doctors and healthcare professionals alike. Easy navigation and quick access to SmPCs means that results that are just one click away.
SmPC menu in Mediately App.
Access To Thousands of Daily Users: The Mediately app is used by more than 213,000 doctors across Europe. With hundreds of new MDs joining every day, you can reach large groups of doctors and make sure the updates are delivered to as many users as possible through one effective channel.
Updates in Real-Time: SmPC needs to be updated in a timely manner to reflect any new data or changes in a drug’s profile. Unlike books and magazines, which are printed and distributed, apps can be updated in real-time. Weekly updates put Mediately at the very top of the reliable resources. This means that when new information becomes available, it can be quickly added to the app, ensuring that users have access to the most up-to-date information at any moment.
Definition of a Summary of Product Characteristics (SmPC)
“An SmPC is a legal document approved as a part of the marketing authorisation of each medicine. It is the basis of information for healthcare professionals on how to use the medicine. Information is updated throughout the life cycle of the product as new data emerge. 4” The structure of an SmPC is defined by the European Medicines Agency and they are used by a range of healthcare professionals (HCPs), including pharmacists, for prescribing and informing on how to use medicine appropriately and safely 5.
Keeping up with changes to SmPCs is essential for HCPs to provide the best possible care to their patients. The summary is organised into six main chapters, including product name, clinical particulars and pharmacological properties.
Wrap up
As long as pharmacology and medicine continue to change and evolve, so will SmPCs. It is the duty of pharmaceutical representatives to successfully use the channels and techniques that are most effective in communicating these updates. And the Mediately mobile app is the perfect tool for this, as it is a trustworthy and independent source of information. Up-to-date content, official databases and accuracy make this mobile assistant one of the most suitable platforms to communicate instant changes and findings in the medical world to doctors.
Sources:
- Mediately. SmPC Updates in Mediately Database Research.
- Mediately Insights. Professionals' Behaviour When Searching for Information About Drugs Used in Breast Cancer and Multiple Sclerosis Treatment Report.
- Mediately. Digital Doctor Report.
- European Medicine Agency. Summary Product Characteristics.
- Freyr Solutions. What is an SmPC.

